RESEARCH
Molecular mechanisms of muscle stem cell activation, proliferation, and self-renewal
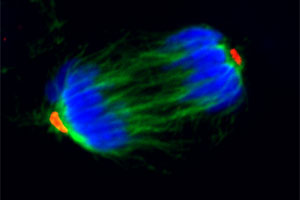
A major interest of the lab is the molecular basis of the transitions of muscle stem cells (satellite cells) between different states.
A major interest of the lab is the molecular basis of the transitions of muscle stem cells (satellite cells) between different states. Specifically, we have focused on the signaling pathways that maintain quiescence, induce activation from a quiescent state, are necessary to maintain a transient amplifying state, induce myogenic lineage progress to a more differentiated mononucleated cell, and control self-renewal and return to quiescence. In this context, we have explored the contributions of various signaling pathways, particularly the Notch and Wnt signaling pathways. Currently, we are focusing on inducible genetic systems to modulate components of those various pathways to test for their specific roles in satellite cell transitions. Current projects include studies of co-activators of the Wnt pathways and the pleiotropic actions of Wnts in adult muscle stem cells. We are also interested in the role of Pax genes in different aspects of satellite cell function. We are studying the differential regulation of expression and degradation of Pax3 and Pax7, the highly homologous proteins that are expressed in the myogenic lineage. Many of our studies of stem cell fate have centered on questions of asymmetric cell divisions. In this context, we have continued our studies of the “Immortal Strand Hypothesis”, exploring both the molecular mechanisms and functional consequences on non-random sister chromatid segregation during satellite cell activation and proliferative expansion.
Aging and stem cell functionality
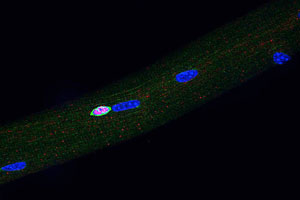
Our lab has used both in vivo and in vitro approaches to explore the underlying basis of changes in stem cell functionality with age, specifically to understand (and perhaps ameliorate) the decline in tissue regenerative capacity with age.
Our lab has used both in vivo and in vitro approaches to explore the underlying basis of changes in stem cell functionality with age, specifically to understand (and perhaps ameliorate) the decline in tissue regenerative capacity with age. Our working hypothesis, based on findings from our laboratory, is that the decline in stem cell functionality with age is primarily due to the aged environment (local niche plus systemic influences) in which stem cells reside and not due to irreversible, intrinsic aging of the stem cells themselves. Whatever intrinsic changes are detected in resting or early activated stem cells may largely be reversible, epigenetic changes. Our studies of aging in the muscle stem cell compartment have shown that a key activator of satellite cells, the Notch ligand Delta-like-1, fails to be induced in aged muscle as compared to young muscle, in response to injury. Current studies include analysis of the regulatory mechanisms of Delta expression and age-related epigenetic changes that may be alter gene expression patterns. We are also pursuing systemic factors that may modulate stem cell functionality based on our findings of heterochronic parabiotic effects, both in terms of the identification of those factors and in terms of how they either enhance or suppress stem cell activity.
Muscular dystrophy pathogenesis and therapy
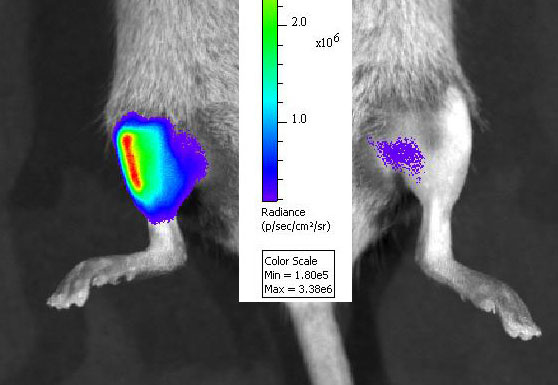
We have a long-standing interest in understanding the mechanisms of cell injury and cell death in the muscular dystrophies and the development of novel therapeutics.
We have a long-standing interest in understanding the mechanisms of cell injury and cell death in the muscular dystrophies and the development of novel therapeutics. In terms of pathogenesis, we have focused on the modulation of cell survival pathways in muscle cells bearing mutations that cause muscular dystrophies in humans. These have brought together the wealth of genetic data with the puzzling heterogeneity of disease expression in different muscle groups and over time. Our studies of therapeutics have focused primarily on non-viral gene therapy modalities, including oligonucleotide-mediated gene repair and plasmid-mediated gene delivery. Current research involves studies of the mechanisms of oligonucleotide actions, and technological developments to enhance the efficiency and efficacy of gene repair. We study these diseases both in vivo using mouse models and in vitro using muscle cell cultures whenever possible. The diseases have focused most on are those involving mutations in the dystrophin gene (Duchenne muscular dystrophy), the caveolin-3 gene (Limb-girdle muscular dystrophy 1C), and the dysferlin gene (Limb-girdle muscular dystrophy 1B). Of note, our research on muscle stem cells is turning toward therapeutic applications. A major emphasis of our recent efforts are pre-clinical studies of using muscle stem cells in models of cell transplantation to treat murine muscular dystrophies with an eye toward future human trials.
Myogenic differentiation
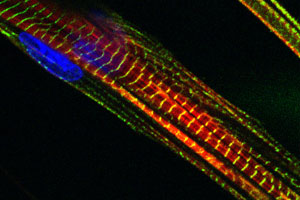
We also have a long-standing interest in the mechanisms of myogenic differentiation, including the fusion of mono-nucleated myoblasts into multinucleated myotubes, a nascent form of the mature muscle fiber.
We also have a long-standing interest in the mechanisms of myogenic differentiation, including the fusion of mono-nucleated myoblasts into multinucleated myotubes, a nascent form of the mature muscle fiber. We have been particularly interested in specific integrin signaling pathways and how they regulate myogenic differentiation and myoblast fusion. Current projects related to the role of the integrin effector molecule, focal adhesion kinase (FAK), in cell fusion. Other ongoing projects include studies of the role of the Deltex family of proteins in the regulation of MyoD expression and myogenic differentiation, the role of the Notch inhibitory protein Numb in regulating the transition from growth to differentiation, and the role of non-canonical Wnt signaling in myogenesis. We have also begun to explore the role of specific microRNA’s in myogenic differentiation and fusion.
